OUR REAGENT ASSAY
ReaGenX delivers high-precision molecular and serological reagent assays, designed to empower CLIA-certified labs with fast, reliable, and scalable diagnostics across UTI, STI, respiratory, wound, and cancer markers.
REAGENX
🦠 RSV + COVID + FLU A/B PANEL
ReaGenX delivers high-precision molecular and serological reagent assays, designed to empower CLIA-certified labs with fast, reliable, and scalable diagnostics across UTI, STI, respiratory, wound, and cancer markers.

Validated for Leading PCR Platforms

Validated for Leading PCR Platforms

Built-In Internal Control for Accurate Validation

Built-In Internal Control for Accurate Validation

Comprehensive Respiratory Panel Detection

Comprehensive Respiratory Panel Detection

Ready-to-Use Lyophilized Reagent System

Ready-to-Use Lyophilized Reagent System

Outbreak Surveillance Ready

Outbreak Surveillance Ready

High-Throughput & Fast Turnaround

High-Throughput & Fast Turnaround
🫁 EXPANDED RSV PATHOGEN PANEL DETECTS SARS‑COV‑2, RSV, ADV, FLU A/B, HPIV
ReaGenX delivers high-precision molecular and serological reagent assays, designed to empower CLIA-certified labs with fast, reliable, and scalable diagnostics across UTI, STI, respiratory, wound, and cancer markers.



High-Coverage Respiratory Pathogen Detection

High-Coverage Respiratory Pathogen Detection

Lyophilized (Freeze-Dried) Mastermix for Workflow Speed

Lyophilized (Freeze-Dried) Mastermix for Workflow Speed

Rapid Results with Scalable Automation

Rapid Results with Scalable Automation

Ideal for Hospitals, Labs & Public Health Use

Ideal for Hospitals, Labs & Public Health Use

Internal Control Ensures Accuracy

Internal Control Ensures Accuracy

Compatible With Major PCR Platforms

Compatible With Major PCR Platforms
🚻 UTI DIAGNOSTIC PANEL
ReaGenX delivers high-precision molecular and serological reagent assays, designed to empower CLIA-certified labs with fast, reliable, and scalable diagnostics across UTI, STI, respiratory, wound, and cancer markers.



UTI + DR MOLECULAR DIAGNOSTIC PANEL

UTI + DR MOLECULAR DIAGNOSTIC PANEL

Rapid Turnaround – Results in ~2 Hours

Rapid Turnaround – Results in ~2 Hours

Integrated Drug Resistance Marker Profiling

Integrated Drug Resistance Marker Profiling

Built-in Internal Control for False Negative Mitigation

Built-in Internal Control for False Negative Mitigation

Broad-Spectrum Pathogen Detection

Broad-Spectrum Pathogen Detection

Ideal for Antimicrobial Stewardship Programs

Ideal for Antimicrobial Stewardship Programs

Lyophilized All-in-One PCR Reagent System

Lyophilized All-in-One PCR Reagent System

High Sensitivity & Automation Compatible

High Sensitivity & Automation Compatible
🧪 STI SCREENING PANEL
ReaGenX delivers high-precision molecular and serological reagent assays, designed to empower CLIA-certified labs with fast, reliable, and scalable diagnostics across UTI, STI, respiratory, wound, and cancer markers.

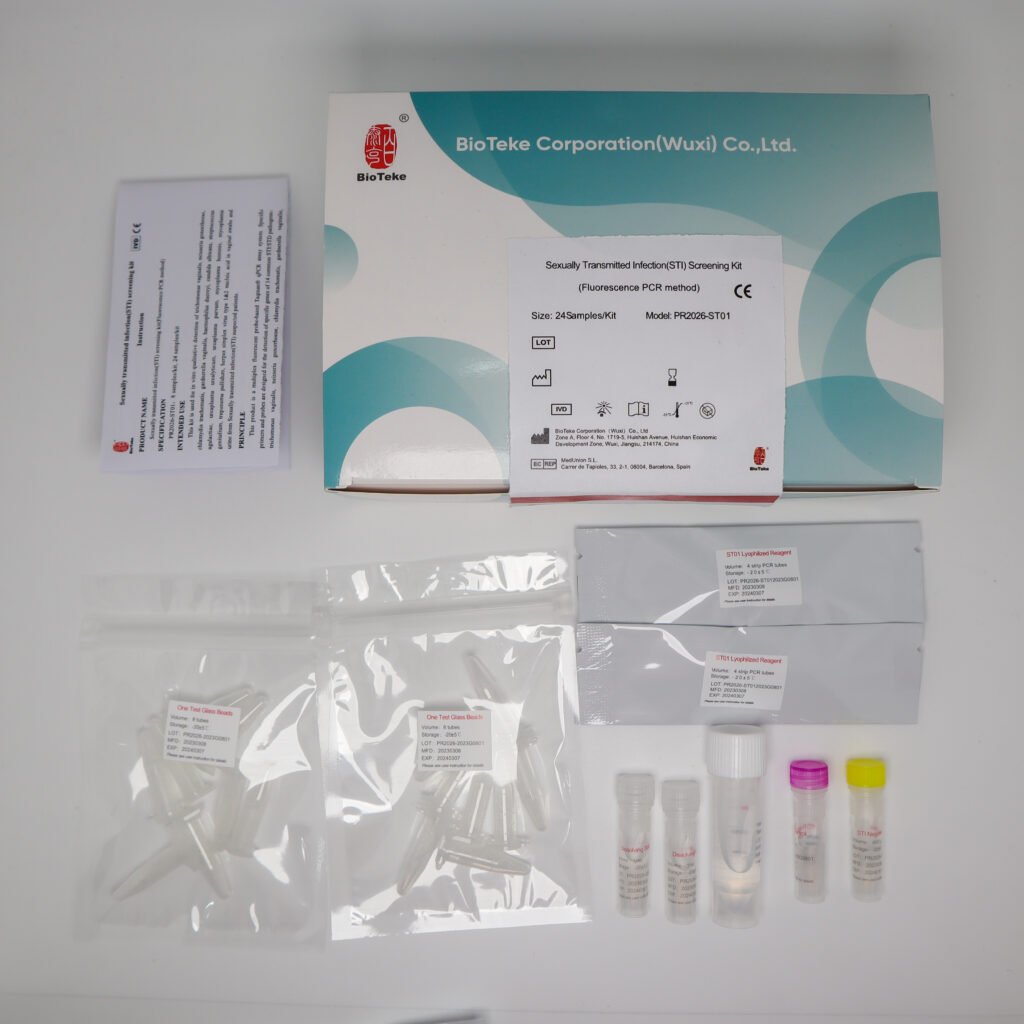

Comprehensive 14-Pathogen STI Panel

Comprehensive 14-Pathogen STI Panel

Lyophilized, Ready-to-Use qPCR Format

Lyophilized, Ready-to-Use qPCR Format

Multiplex qPCR with High Specificity

Multiplex qPCR with High Specificity

Internal Control (RNaseP) for Every Reaction

Internal Control (RNaseP) for Every Reaction

Ideal for Clinics, Labs & Public Health

Ideal for Clinics, Labs & Public Health

Fast Turnaround with PCR Compatibility

Fast Turnaround with PCR Compatibility
🩹 WOUND INFECTION PATHOGEN PANEL
ReaGenX delivers high-precision molecular and serological reagent assays, designed to empower CLIA-certified labs with fast, reliable, and scalable diagnostics across UTI, STI, respiratory, wound, and cancer markers.
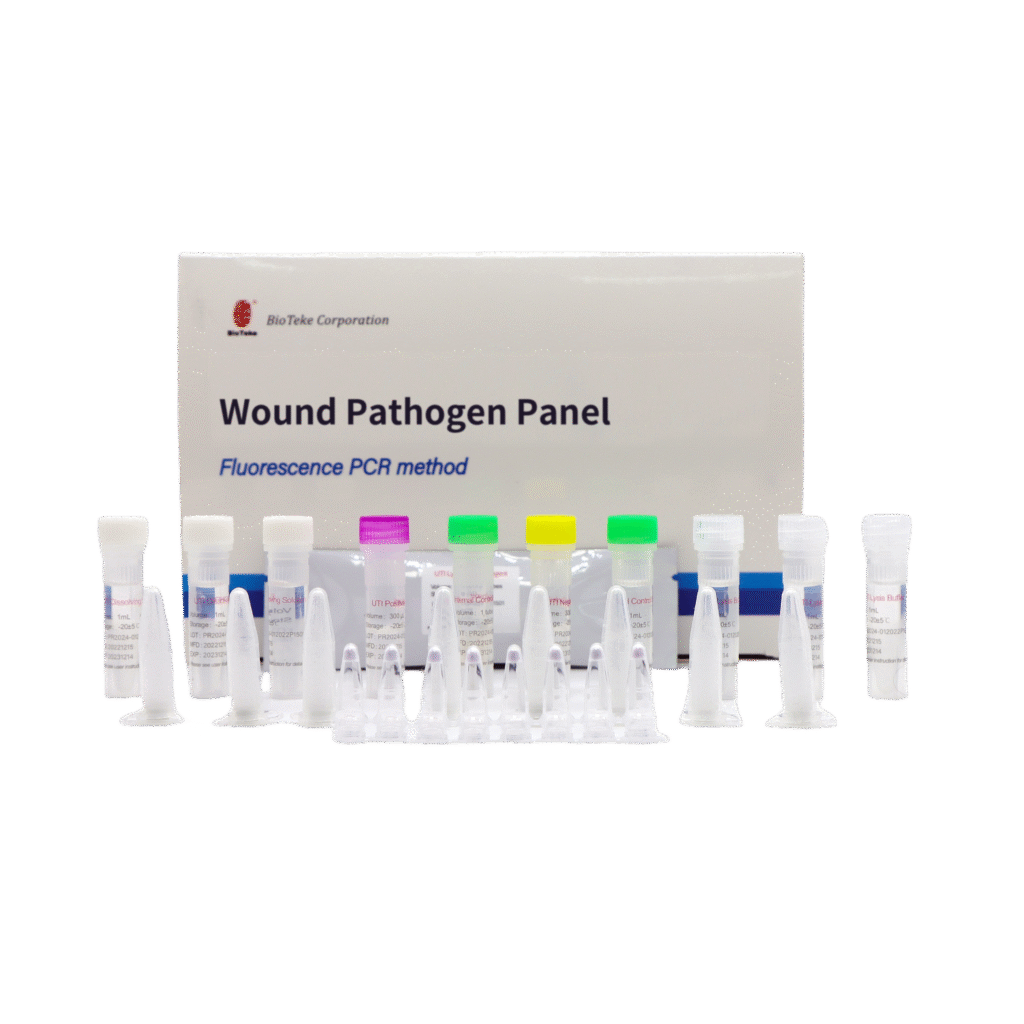

Broad-Spectrum Pathogen Coverage

Broad-Spectrum Pathogen Coverage

12 AMR Gene Targets in One Test

12 AMR Gene Targets in One Test

Lyophilized All-in-One PCR System

Lyophilized All-in-One PCR System

Internal Control & PCR Contamination Control

Internal Control & PCR Contamination Control

High Sensitivity & Specificity

High Sensitivity & Specificity

Compatible With Leading PCR Platforms

Compatible With Leading PCR Platforms

Automation-Ready Workflow

Automation-Ready Workflow

Fast Turnaround for Urgent Wound Management

Fast Turnaround for Urgent Wound Management
🧬 CUSTOM PCR PANEL DEVELOPMENT
Tailored Multiplex Solutions Built Around Your Lab’s Needs


1. Expert-Led Panel Design

1. Expert-Led Panel Design

2. Lyophilized, Ready-to-Use Format

2. Lyophilized, Ready-to-Use Format

3. Cross-Platform Compatibility

3. Cross-Platform Compatibility

4. Rapid Development Timeline

4. Rapid Development Timeline

5. OEM / White Label Options

5. OEM / White Label Options

6. Regulatory-Ready Documentation

6. Regulatory-Ready Documentation

7. Sample Type Flexibility

7. Sample Type Flexibility

8. Flexible Batch Sizes for R&D or Clinical Scale

8. Flexible Batch Sizes for R&D or Clinical Scale
OUR REAGENT ASSAYS
RSV
The ReagenX™ RSV-X Multiplex PCR Kit is intended for the qualitative in vitro detection of nucleic acids from key respiratory pathogens — including Severe Acute Respiratory Syndrome Coronavirus 2 (2019-nCoV, ORF1ab and N genes), Influenza A virus, Influenza B virus, and Respiratory Syncytial Virus (RSV A/B). The Human RNaseP gene is included as an internal control to verify specimen integrity and amplification validity. The assay is designed for use with patients suspected of respiratory infection, including those with suspected clustered cases or other individuals requiring rapid respiratory pathogen detection, using real-time PCR.
RSV+
The ReagenX™ RSV+ Multiplex PCR Kit is intended for the qualitative in vitro detection of nucleic acids from 29 common respiratory pathogens, including viruses, bacteria, and fungi. Detected pathogens include:
Viruses: Severe Acute Respiratory Syndrome Coronavirus 2 (2019-nCoV), Influenza A virus, Influenza B virus, Respiratory Syncytial Virus (RSV A/B), Respiratory Adenovirus, Human Rhinovirus, Human Metapneumovirus, Human Bocavirus, Parainfluenza viruses type I–IV, and Coronaviruses 229E, OC43, NL63, HKU1.
Bacteria: Klebsiella pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumoniae, Streptococcus pneumoniae, Legionella pneumophila, Bordetella pertussis, Haemophilus influenzae, Streptococcus pyogenes (Group A), Chlamydia psittaci.
Fungi: Aspergillus spp., Cryptococcus spp., and Pneumocystis jiroveci. ABI 7500, Bio-Rad CFX96, QuantStudio, SLAN-96S, BTK-96

UTI
Qualitative detection of 31 genetic targets associated with wound infections, including 19 bacterial and fungal pathogens and 12 antimicrobial resistance genes.
• Pathogens (19): Escherichia coli, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Citrobacter spp., Enterobacter cloacae, Acinetobacter baumannii, Proteus mirabilis, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus agalactiae, Enterococcus faecalis, Enterococcus faecium, Morganella morganii, Bacteroides fragilis, Staphylococcus epidermidis, Candida albicans
• Resistance Genes (12): blaKPC, blaNDM, blaIMP, blaVIM, blaOXA-48, mecA, vanA, vanB, CTX-M2, sul1, sul2, sul3
• Specimen Types: Wound swabs or aspirates Internal Control: Yeast DNA for extraction and amplification monitoring.

STI
The ReagenX™ STI-X Multiplex PCR Kit is intended for the qualitative detection of nucleic acids from 14 pathogens associated with sexually transmitted infections, including Trichomonas vaginalis, Neisseria gonorrhoeae, Chlamydia trachomatis, Gardnerella vaginalis, Haemophilus ducreyi, Candida albicans, Streptococcus agalactiae, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, Mycoplasma genitalium, Treponema pallidum, Herpes simplex virus type 1, and Herpes simplex virus type 2 in vaginal swab and urine specimens, using multiplex real-time fluorescent PCR.
WOUND
Qualitative detection of 31 genetic targets associated with wound infections, including 19 bacterial and fungal pathogens and 12 antimicrobial resistance genes.
• Pathogens (19): Escherichia coli, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Citrobacter spp., Enterobacter cloacae, Acinetobacter baumannii, Proteus mirabilis, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus agalactiae, Enterococcus faecalis, Enterococcus faecium, Morganella morganii, Bacteroides fragilis, Staphylococcus epidermidis, Candida albicans
• Resistance Genes (12): blaKPC, blaNDM, blaIMP, blaVIM, blaOXA-48, mecA, vanA, vanB, CTX-M2, sul1, sul2, sul3
• Specimen Types: Wound swabs or aspirates
• Internal Control: Yeast DNA for extraction and amplification monitoring
BROCHURE
OTHER PRODUCTS
8-in-1 Respiratory Antigen Rapid Test Kit – RES-8-1

Helicobacter pylori (H. pylori) Antigen Rapid Test Kit – GAS-1

Neuron-Specific Enolase (NSE) Rapid Test Kit – URO-2

Urine hCG Pregnancy Rapid Test Kit – PRG-1U

Canine Influenza Virus (CIV) Rapid Test Kit – VET-1
Dengue NS1/IgG/IgM Rapid Test Kit – TRP-1

Instrux™ Nucleic Acid/Virus Aerosol Removal System – NC1001

Instrux™ Indoor Air Purifier & Disinfector – DS600

InstruX™ Nucleic Acid Extractor – 96 Throughput

InstruX™ Micro-volume UV-Vis Spectrophotometer

InstruX™ Ultra-Fast RT-PCR Thermal Cycler – BTK-8

Instrux™ Automated Sample Transfer Processing System