- WOUND
ReagenX
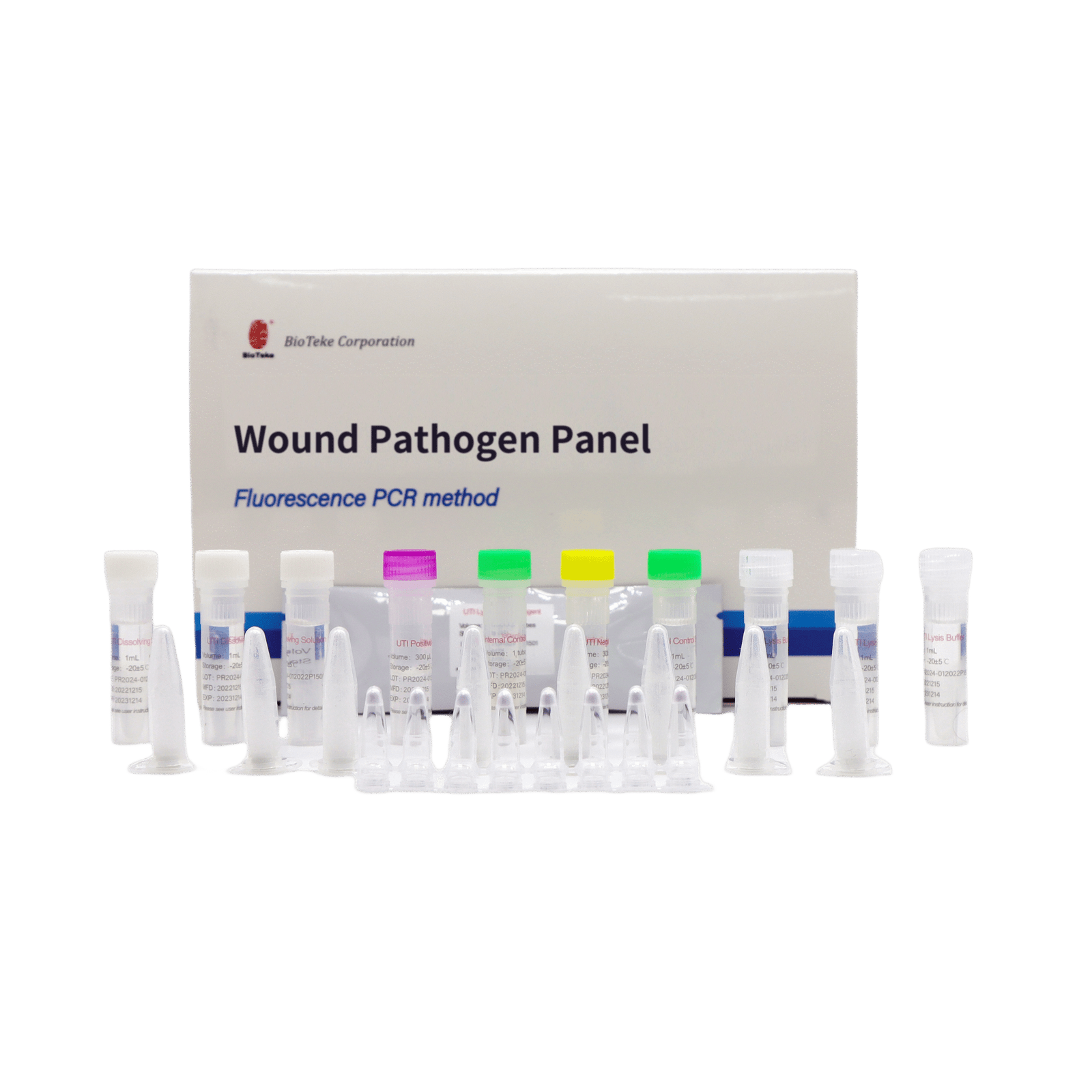

Wound Pathogen Panel (Fluorescence PCR method)
A multiplex qPCR panel designed for rapid detection of bacterial, fungal, and antimicrobial-resistance targets directly from wound swabs. Provides comprehensive profiling of 31 genetic markers to support accurate infection management and targeted therapy. Room-temperature-stable reagents simplify storage and shipping without cold-chain requirements.
The kit is designed with specific primers and probes for different common pathogens and drug resistance genes. Polymerase chain reaction (qPCR) and multiple fluorescent probe technique are used to amplify and detect specific nucleic acid sequences of pathogens and drug resistance genes listed in Table 1.
By adding edible yeast as internal control (IC), the extraction and detection process of pathogen nucleic acid from wound swab is monitored to effectively avoid false negative results. In order to avoid aerosol contamination of the amplified products, the UDG enzyme /dUTP system was added to the amplification system to effectively degrade the amplified products and avoid false positive results.
This kit is a fully premix freeze-dried system. Taq enzyme, UDG enzyme, reaction buffer, specific primers and probes required for amplification are all lyophilized in PCR tubes. A total of 1-8 Wells are lyophilized powders for different target genes. Detection can be performed directly after adding dissolving solution and extracted nucleic acid.
Model: PR2028-WP01
Format: Test cassette
Specimen: Wound swab
Storage: -25~-15℃(-13~5℉)
Shelf life: 12 months
Wound Infection (WI) Pathogen Panel – Kit Components
| Components | 8 samples/kit | 24 samples/kit | Ingredient |
|---|---|---|---|
| Wound Lyophilized Reagent | 8×8 strip tubes | 24×8 strip tubes | Specific primer&probes for the detection of pathogens and DR genes in Table 1, dNTP/dUTP Mix, Mg2+, Taq polymerase and UDG enzyme |
| Internal Control Dry Powder | 1 tube | 1 tube | Edible yeast powder |
| Internal Control Solution | 1 mL×1 tube | 1 mL×1 tube | Dnase/Rnase-free H2O |
| Wound Positive Control | 300 μL×1 tube | 300 μL×1 tube | Plasmid containing every target gene sequence |
| Wound Negative Control | 300 μL×1 tube | 300 μL×1 tube | Plasmid containing internal control sequence |
| Dissolving Solution | 1 mL×1 tube | 1 mL×3 tubes | Stabilizer |
NOTE:
1. Do not mix the components from different batches for detection.
2. The kit can be transported at room temperature (no more than one month).
3. Repeated freezing and thawing should not exceed 7 times.
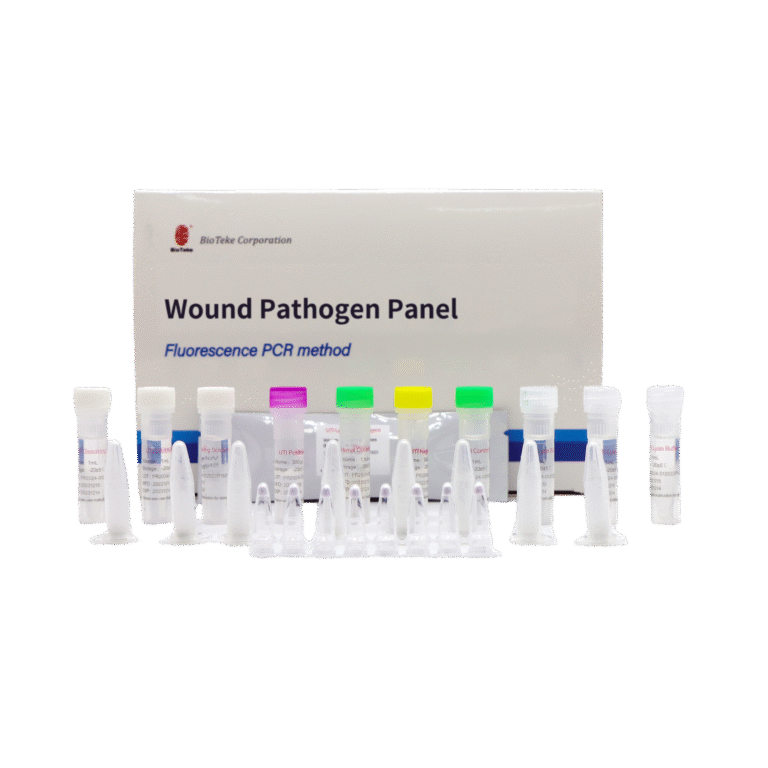
1. Multiple quantitative analysis
19 common pathogen nucleic acids and 12 common antibiotics resistance genes
2. Rapid response
With a fast PCR instrument, the test can be completed in 60 minutes
3. Application scenarios
Urology routine testing/third-party testing laboratory/inspection department
Postoperative monitoring/disease control/scientific research units/doctors cooperate to publish articles
4. Product advantages
Freeze-dried system
Reduce transportation risks and increase stability
No extraction equipment required
1. Disposable powder free gloves and other personal protective equipment;
2. Pipettes (adjustable) and Sterile pipette tips;
3. Wound swab collection device;
4. 1.5 mL centrifuge tubes and racks;
5. Bench top centrifuge for centrifuge tubes and PCR tubes;
6. Bacterial nucleic acid extraction kit(It is recommended to use Nucleic Acid Extration Reagent manufactured by Bioteke, Item No.: BUR01);
7. Nucleic acid extractor;
8. Metal bath/water bath (1.5mL centrifuge tube,95℃);
9. Real-time PCR instrument with FAM/VIC/ROX/CY5 fluorescence channels(ABI7500,Bio-rad CFX96,QuantStudio,SLAN-96S,BTK-96).
2). Precision: (CV, %) of Ct ≤5%.
3). Accuracy: The conformity rate of negative/positive reference 100%.
4). Specificity: Please refer to the User Manual.
RELATED PRODUCTS
RSV Multiple Respiratory Pathogens Nucleic Acid Detection Kit (Fluorescence PCR method)
RSV+ Extended Respiratory Pathogen Panel (RSV plus influenza, adenovirus, parainfluenza, coronaviruses)

Sexually Transmitted Infection (STI) Screening Kit (Fluorescence PCR method)

Urinary Tract Infection (UTI) & DR Panel (Fluorescence PCR method)
Wound Pathogen Panel (Fluorescence PCR method)
OTHER PRODUCTS
8-in-1 Respiratory Antigen Rapid Test Kit – RES-8-1

Helicobacter pylori (H. pylori) Antigen Rapid Test Kit – GAS-1

Neuron-Specific Enolase (NSE) Rapid Test Kit – URO-2

Urine hCG Pregnancy Rapid Test Kit – PRG-1U

Canine Influenza Virus (CIV) Rapid Test Kit – VET-1
Dengue NS1/IgG/IgM Rapid Test Kit – TRP-1

Instrux™ Nucleic Acid/Virus Aerosol Removal System – NC1001

Instrux™ Indoor Air Purifier & Disinfector – DS600

InstruX™ Nucleic Acid Extractor – 96 Throughput

InstruX™ Micro-volume UV-Vis Spectrophotometer

InstruX™ Ultra-Fast RT-PCR Thermal Cycler – BTK-8

Instrux™ Automated Sample Transfer Processing System